
Illustration of tree, cones, and foliage [Matt Strieby, 2020].

Bark. Top left, an 80 cm dbh tree in savanna at Cortes Pass near Popocatepetl. Top right, a 60 cm dbh tree on Nevado de Colima. Bottom left, a 100 cm dbh tree in continuous montane forest at Nevado de Toluca. Bottom right, a 60 cm dbh tree in continuous montane forest at Nevado de Toluca. All photos by C.J. Earle, 2005.02.

Foliar units and branchlets on a tree at Nevado de Toluca [C.J. Earle, 2005.02.12].

Cone collected on east side of Cortes Pass. Scale is in cm [C.J. Earle, 2005.02.05].

Cone in situ, Cerro Potosí [C.J. Earle, 2007.02.19].

Unopened cones in situ on a tree at Nevado de Colima [C.J. Earle, 2005.02.14].

Seedling, part of an extensive regeneration patch near timberline on Nevado de Toluca. The stout stem and profusion of needles suggests this seedling is at least several years old [C.J. Earle, 2005.02.14].
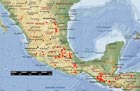
Distribution of P. hartwegii. Red circles indicate specimens examined by Farjon and Styles (1997); pink areas and circles indicate limits of distribution according to Critchfield and Little (1966) and Perry (1991). Basemap from Expedia Maps.

Pinus hartwegii and Nevado de Colima. Apart from a few scattered Abies religiosa, every tree in this photograph is P. hartwegii [C.J. Earle, 2005.02.14].

An open stand on Cerro Potosí [C.J. Earle, 2007.02.19].

Continuous pure forest about 6 km east of Cortes Pass on the slopes of Popocatepetl. Uniform canopy height and tree size, and monotonous dominance of a grass understory, are typical of montane stands of this species [R. Van Pelt, 2005.02.05].

Trees growing in exposed setting on the Cerro Potosí summit ridge; shrubby short-needled pine at left is P. culminicola [C.J. Earle, 2007.02.19].

Timberline forest on the northeastern slopes of Nevado de Toluca. The transition from forest to nonforest is fairly abrupt, but there are erect-growing trees at elevations well above the continuous forest. An aspect effect is apparent on this mountain; south-facing timberlines are higher, suggesting that timberline is, as usual, a function of temperature [C.J. Earle, 2005.02.12].

"Pom-pom" growth form on a tree ca. 2 m tall at the north pass on Highway 175 in Oaxaca. Technically these are timberline trees, growing over a shrubby ericad understory [C.J. Earle, 2005.02.08].

Tree scarred for resin collection, east of Cortes Pass [C.J. Earle, 2005.02.05].

Mistletoe growing on P. hartwegii, Cerro Potosí [C.J. Earle, 2007.02.19].

The only pine that grows at elevations higher than 4000 m is P. hartwegii, here seen at 4200 m on Nevado de Toluca in Mexico [C.J. Earle, 2005.02.12].

Pinus hartwegii
Lindley 1839
Common names
Pino de México, ocote, pino, pino escobetón, pino negro [Spanish]; Mexican mountain pine, Hartweg's pine.
Taxonomic notes
Syn: P. montezumae Lamb. subsp. hartwegii (Lindl.) Engelm. 1880, P. montezumae var. hartwegii (Lindl.) Shaw 1909, P. rudis Endl. 1847, P. montezumae Lamb. var. rudis (Endl.) Shaw 1909, P. hartwegii Lindl. var rudis (Endl.) Silba 1990, P. lindleyana Gordon 1858, P. montezumae Lamb. var. lindleyana (Gordon) Parl. 1868, P. donnell-smithii Mast. 1891, and a long slate of species described by Roezl in an 1857 catalog and never heard from again; see Farjon and Styles (1997) for the complete listing, and see Pinus montezumae for remarks on Roezl's creative approach to pine systematics.
It is understandable that such a plethora of names have been attributed to this taxon; it does seem odd that a single species of pine dominates the subalpine forests from Nuevo León to El Salvador, an area within which there are no large contiguous subalpine forests and every range is a biogeographical island; but despite many attempts to subdivide the taxon, none have succeeded in identifying the kind of regional morphological distinctions that credibly support the creation of other taxa at the specific or varietal ranks.
Genetic studies (see the "Taxonomic notes" for Pinus ponderosa for further discussion) place this taxon in Subgenus Pinus, Section Trifoliae, Subsection Ponderosae. The other 13 species in the subsection are widely-distributed habitat generalist pines of western North America; most of them are phenotypically plastic. Most are taxa of mountainous regions and are comprised of a large number of geographically distinct populations that have opportunities to merge and mingle as a consequence of climate changes (glacial/interglacial, mainly) that primary occur at time scales of tens to hundreds of thousands of years. Consequently we would expect P. hartwegii to be a phenotypically and presumably genetically diverse taxon that sometimes displays consistent morphological differences between populations, which may be assignable to subspecific or varietal ranks.
Historically, the taxon was separately described at several times, owing to its broad geographic distribution. In 1839, Lindley described P. hartwegii from material collected by C.T. Hartweg in the mountains of eastern Michoacán, Mexico. Then in 1847, Endlicher described P. rudis, and in 1891, Masters described P. donnell-smithii from material collected by Donnell-Smith in Guatemala. Unfortunately, only Donnell-Smith's collection survives; Hartweg's specimen has been lost, and we do not even know the origin of the material described by Endlicher. Later authors did not clarify matters. Martínez distinguished the species based on cone color, which is not generally regarded as a taxonomically useful character, and the character states described by Perry (1991) for the different taxa are overlapping or even indistinguishable (Farjon and Styles 1997). Farjon and Styles (1997) cite a study by Matos (1995) that was unable to discriminate P. hartwegii and P. rudis using elevational transects recording 25 character states, and they conclude that the taxa, as described, are undistinguishable. Thus they reduce all names since the original P. hartwegii to synonymy.
At this point I have seen this taxon on Cerro Potosí and Cerro Peña Blanca in Nuevo Leon, on Nevado de Colima in Jalisco, in the Sierra Juárez in Oaxaca, on Nevado de Toluca in México, and on Popocatepetl in Puebla. This represents a pretty good cross-section of its distribution in Mexico. I have not seen it in Guatemala or Honduras, which are the described range of P. donnell-smithii. Based on these observations I agree with Farjon and Styles that the taxon described by Perry (1991) as P. rudis is not distinguishable from P. hartwegii. However, Perry (1991) was clearly seeing what he thought to be consistent differences between trees in different areas. I also received an email from Burkhard Witt (2007.02.04) describing his field observations, using Perry (1991) as a guide, on numerous high mountains in northeast Mexico, and concluding that P. rudis and P. hartwegii, as described by Perry, are differentiable and consistently occur with P. rudis at lower elevations, giving way to P. hartwegii at higher elevations (see the Observations section). Witt also reported that he has observed a consistent difference in that P. rudis consistently has a silver-gray-green foliage color, while P. hartwegii has very dark green foliage. This is interesting information, but doesn't get around the taxonomic problems that the original descriptions of these species are not adequate to differentiate them, and that Perry's keys do not effectively discriminate between the taxa. I am forced to conclude that there probably is a sound basis for distinguishing subspecies or varieties within P. hartwegii. Maybe someday some enterprising taxonomist will go out and systematically study populations representing the range of the taxon, and will describe taxa that represent patterns of variation that are not reflected in the published descriptions of P. rudis or P. donnell-smithii. Until then, if you go in search of these trees, it is well to look out for evidence of infraspecific differences that may be tied to elevation or geography.
Description
A tree up to 128 cm dbh and 31 m tall, almost always arboriform even at the alpine timberline. Bark thick, rough and scaly, divided into small to large plates, deeply furrowed, dark brown to grey. Branchlets stout, stiff, curving upwards, purple-brown turning dark brown or grey, with persistent leaf bases. Needles in fascicles of 3-6, usually 5 but mostly 3 in some areas (e.g. Popocatepetl), (6-)10-17(-22) cm long × (1-)1.2-1.5 mm, straight or curved, stiff. Cones in whorls of 2-6, appearing sessile, deciduous, obliquely ovoid, 8-12(-15) × 5-8 cm when open. Cones scales opening soon, with a slightly raised apophysis, weakly transversely keeled, brown or purple-brown with a black flat umbo. Seeds 5-6 mm long, often with black spots; seed wing articulate, 12-20 × 7-12 mm (Farjon et al. 1997 and personal observations in southern Mexico, Feb-2005). See García Esteban et al. (2004) for a detailed characterization of the wood anatomy.
Distribution and Ecology
- Guatemala: departments of Chimaltenango, El Quiché, Guatemala, Huehuetenano, Quezaltenango, Sacatepequez, San Marcos, Sololá, and Totonicapán.
- Honduras: on Cerro Santa Bárbara
- Mexico: states of Chiapas, Chihuahua, Coahuila, Colima, Distrito Federal, Durango, Guerrero, Hidalgo, Jalisco, México, Michoacán, Morelos, Nuevo León, Oaxaca, Puebla, Tamaulipas, Tlaxcala, and Veracruz
Distribution data from USGS (1999).
Found at elevations of (2200-)2500-4000(-4389) m (Perry 1991, Farjon et al. 1997). Also reported from El Salvador, Perry (1991) asserting that "there are still many relatively unexplored high mountains along the Honduras-El Salvador border and P. hartwegii, along with other unreported species, may occur on those mountain peaks." Hardy to Zone 8 (cold hardiness limit between -12.1°C and -6.7°C) (Bannister and Neuner 2001). See also Thompson et al. (1999).
From central Mexico southwards, this is the universal pine at high altitudes, where it normally grows erect to the upper timberline in pure (single-species) stands, though at more moderate elevations it may be found with Pinus montezumae, P. pseudostrobus, P. ayacahuite, Abies religiosa and Hesperocyparis lusitanica. These forests typically have a simple structure, with an open, park-like overstory, and an herb-bunchgrass understory (Velázquez et al., 2000). Data from a site on Nevado de Colima show that the climate is characterized by dry winters and a wet June to October monsoon, with sub-freezing temperatures from July through March (Biondi et al. 2005). At the alpine timberline its growth may be primarily limited, on a year-to-year basis, by the timing of the onset of warm spring temperatures and, later in the season, by moisture stress; additionally, these upper timberline sites are commonly subjected to intense grazing and frequent fires. These factors may help to explain why this species does not attain the dwarfed, contorted alpine growth form known as krummholz -- the species, though growing at the alpine timberline, does not experience the adverse effects of cold and wind-blown ice that normally cause the krummholz form. In this connection, it is worth noting that this is the only pine in the world that grows at elevations higher than 4000 m, reaching a maximum of 4389 m on the slopes of Nevado de Toluca in Mexico. Pinus hartwegii has recently been the subject of global comparative studies on treeline ecophysiology and biogeography (Hoch and Körner 2003, Körner and Paulsen 2004).
This species is afflicted by the round-headed pine beetle, Dendroctonus adjunctus, a bark beetle that can cause high incidence of mortality in susceptible stands (Hartsough and Biondi 2004, Biondi et al. 2005). In southern Mexico and Guatemala, it is also a host to the dwarf mistletoe Arceuthobium globosum subsp. grandicaule, and A. vaginatum subsp. vaginatum throughout much of Mexico (Hawksworth and Wiens 1996).
Remarkable Specimens
There are not a lot of data. Biondi (2001) sampled trees up to 128 cm dbh on Nevado de Colima, and I (2005.02) got laser-measured heights up to 30.5 m tall on Nevado de Toluca. The oldest known tree was a specimen collected at 19.58° N, 103.62° W, elevation 3,700 m, on Nevado de Colima; its ring-width record began in 1553 (Biondi 2001).
Ethnobotany
This tree is commonly scarred for resin collection. Such resin collection is typically performed on a non-industrial scale, marking isolated trees, with the resin used for caulk and other purposes not requiring refinement or distillation.
The species has been useful in dendrochronology. Franco Biondi and his students have been doing chronology development and physiological studies at Nevado de Colima since 1999 (maybe before). Biondi (2001) collected a 400-year chronology on the peak. This chronology shows the growth suppression due to the 1913 Volcan Fuego eruption, as well as growth suppression due to the catastrophic 1815 eruption of Tambora in Indonesia. Interestingly, the chronology shows a strong correlation with the Palmer Drought Severity Index for the central Great Plains of the United States, suggesting that the intensity of the summer monsoon is a primary control on tree growth at this alpine site. Biondi and others (2003) found that a 1913 eruption of Volcán Fuego, Colima's sister peak, produced reduced growth rings for the following two years in trees around Colima, and more recent work documented the effect of a bark beetle outbreak on growth of these trees (Biondi et al. 2005).
Observations
I have seen it in six locations, all but one remarkable and easily accessible:
- Parque Nacional Iztaccíhuatl Popocatépetl. This site is accessible, I believe, by public buses from Amecameca. It is a very extensive forest of P. hartwegii, running for about 20 km along the road over Cortés Pass and extending to the upper timberline on Iztaccíhuatl and Popocatépetl, virtually all of it as a pure stand over a grass understory.
- Highway 175 in Oaxaca. The site is accessible by buses crossing highway 175 from Oaxaca to Tuxtepec. At this low latitude apparently no other trees can effectively compete even at an elevation of only 3,000 meters, and what would at a more northerly site be a montane forest is here a nearly pure stand of stunted timberline pines (with a few Pinus ayacahuite mixed in), over a diverse shrub understory. The photos at right show the unusual "pom-pom" growth form of these trees.
- Nevado de Toluca. The site is accessible by tour buses running from Toluca and Mexico City. From the park entrance onwards, P. hartwegii is the only pine you will see on this mountain, and the only other conifer is represented by a few shrubby Juniperus monticola growing well above timberline. Although the road follows the timberline for many kilometers, eventually climbing into the very crater of the volcano, still there are nowhere old or krummholz form trees, a testimony to the frequently disturbed character of this treeline. I have been told that grazing and anthropogenic fire are the principal disturbances at this timberline.
- Nevado de Colima. The site is accessible by occasional tour buses from Colima or the coastal resorts. This site is the focus of dendrochronological research on the species and is in any event a prime attraction for conifer lovers, having I believe the highest conifer species diversity of any comparably sized area in Mexico. On the drive up the mountain you will see, with increasing elevation, Pinus devoniana, P. maximinoi, P. leiophylla, P. montezumae, P. pseudostrobus, Abies religiosa, P. ayacahuite, P. hartwegii, and Juniperus monticola. Elsewhere on the mountain there are reported to be Abies guatemalensis, Hesperocyparis lusitanica, Pinus douglasiana, P. herrerae, P. oocarpa, P. teocote, a small outlying population of P. durangensis, and Podocarpus matudae.
- Cerro Peña Nevada. The site is accessible only by a long and very rough road out of Doctor Arroyo, Nuevo León. The area I saw is just barely high enough to be in the range of the species. It's a great trip, but not as a place to see P. hartwegii.
- Cerro Potosí. The site is accessible if you have a motorcycle or fairly rugged vehicle, such as a typical two-wheel-drive pickup truck. Because it is a park and a popular tourist destination, it is likely that tours run there from Saltillo or Monterey. The species is widely distributed on the mountain, first occurring with Pinus arizonica and P. strobiformis, continuing upwards to occur on the summit with the extremely rare pine P. culminicola. This is a remarkable site, well worth the effort to get there.
Burkhard Witt (email 2007.02.04) relates the following information about finding P. hartwegii and trees that fit Perry's 1991) description of P. rudis:
- Cerro Peña Nevada, Nuevo León: P. rudis occurs above 2900 m; by 3400 m, almost all trees are P. hartwegii.
- Cerro Potosí, Nuevo León: P. rudis occurs from 2700 m to 3100 m; by 3400 m, all trees are P. hartwegii, continuing up to the summit (about 3710 m).
- Sierra La Marta and Sierra El Coahuilon, Coahuila: In the valley at 2800 m to 2900 m are trees of P. rudis, some of them in a plantation of P. sylvestris. The higher montane forests, up to about 3400 m, were destroyed by fire in 1975 (Perry 1991). P. hartwegii grows between 3400 m and the summit (about 3630 m).
- Cerro El Nacimiento (between Miquihuana and the little settlement Valle Hermoso), Tamaulipas: On the dirt road to "El Aserradero" is a forest of P. rudis at 2900 m to 3100 m, above scattered stands of P. stormiae (2600 m to 2700 m). On the northern slopes of Cerro El Nacimiento, P. rudis grows from 2800 m to the summit (3190 m) appearing as scattered trees, and P. hartwegii is absent.
Totonicapán forest is said to harbor the largest and best-conserved stand of P. hartwegii in Guatemala (Elias 1997).
Remarks
The epithet honors botanist Karl Theodor Hartweg, who collected the type specimen on behalf of the London Horticultural Society. He is remembered in the names of many plants of American arid regions.
Citations
Biondi, F. 2001. A 400-year tree-ring chronology from the tropical treeline of North America. Ambio 30:162-166.
Biondi, F., P.C. Hartsough, and I.G. Estrada. 2005. Daily Weather and Tree Growth at the Tropical Treeline of North America. Arctic and Alpine Research 37:16-24.
Biondi, F., I.G. Estrada, J.C.G. Ruiz, and A.E. Torres. 2003. Tree growth response to the 1913 eruption of Volcán de Fuego de Colima, Mexico. Quaternary Research 59:293-299.
Elías, S., 1997. Autogestión comunitaria de recursos naturales, estudio de caso en Totonicapán. Facultad Latinoamericana de Ciencias Sociales. Guatemala [cited in ParksWatch 2004].
Lindley, J. 1839. Edwards's Botanical Register 25:62.
Hartsough, Peter and Franco Biondi. 2004. High Elevation Monitoring in the North American tropics: Ecosystem/climate relationships on Nevado de Colima, Mexico. Poster presented at the 2004 meeting of the American Geophysical Union, http://woods.geography.unr.edu/Posters/AGU2004%20poster.pdf, accessed 2006.12.31, now defunct.
Hoch, G. and C. Körner. 2003. The carbon charging of pines at the climatic treeline: a global comparison. Oecologia 135:10-21.
Körner, C. and J. Paulsen. 2004. A world-wide study of high altitude treeline temperatures. Journal of Biogeography 31:713-732.
Lindley. 1839.
Matos, J.A. 1995. Pinus hartwegii and P. rudis: A critical assessment. Systematic Botany 20: 6-21.
Velázquez, A., V. M. Toledo, and I. Luna. 2000. Mexican temperate vegetation. Peges 573-592 in M.G. Barbour and W.D. Billings (eds.), North American Terrestrial Vegetation. New York: Cambridge University Press.
See also
Farjon & Styles 1997.
Hartsough, Peter and Franco Biondi. 2003. The Importance of High Elevation Monitoring in a Tropical Tree line
Environment: A Project Update from South Western Mexico. P. 18 in Programme with Abstracts, Fourth Annual Science Meeting, IAI CRN 03: The Assessment of Past, Present and Future Climate Variability from Treeline Environments. IANIGLA-CRICYT, Mendoza, Argentina, October 10-16, 2003.

















