Cephalotaxus
Siebold et Zuccarini ex Endlicher 1842
Common names
Plum yew, 粗榧 cu fei [Chinese], イヌガヤ属 inugaya zoku [Japanese].
Taxonomic notes
A genus of, in this treatment, 8 species (note that as of August 2024 this account and C. sinensis are undergoing revision):
For most of the 20th Century this genus was generally treated as the sole genus in the family Cephalotaxaceae Neger (1907), and some authors also placed Amentotaxus (Taxaceae) in the family. Detailed embryological studies by Singh (1961) showed that Cephalotaxus development differs strongly from all genera in the Taxaceae save Amentotaxus, which exhibits some intermediate characters. Molecular methods were brought to the problem by Hao et al. (2008), using both chloroplast and nuclear DNA markers to analyze all generally recognized species in the two groups, showed that the clade of Cephalotaxus with other Taxaceae genera is monophyletic. However, a molecular (chloroplast genome) analysis by Majeed et al. (2019) reached a very different conclusion, identifying one clade of Amentotaxus, Cephalotaxus, and Torreya, and a second clade of Austrotaxus, Pseudotaxus and Taxus. However, I am skeptical of this study as it did not sequence the whole plastome (see Wang et al. 2022 for why this is a problem in Cephalotaxus), it only used one sample for each species, and it only sequenced 8 species out of the 35 species in these genera. As currently understood, Taxaceae without Cephalotaxus is paraphyletic. It might be appropriate to describe Cephalotaxus as a subfamily of Taxaceae, but that has not yet been done.
Cephalotaxus is an ancient genus, with a recognizable precursor, Thomasiocladus zamioides, dating to the mid-Jurassic and the earliest species of Cephalotaxus dating to the early Cretaceous (Shi et al. 2010). The genus was distributed across much of the northern hemisphere during the Tertiary, with fossil evidence known from Asia, Europe, and North America (Manchester et al. 2009; Fig. 5 in Shi et al. 2010). A molecular clock based on plastid protein-coding genes indicates that all extant species of Cephalotaxus have diversified during Neogene time, with episodes of speciation near the Miocene/Pliocene transition and during the Pleistocene (Ji et al. 2021).
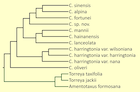
There has been considerable disagreement on the number and identification of taxa in the genus (cf. Table 1 in Wang et al. 2022). Nearly all authorities have agreed in treating C. fortunei, C. hainanensis, and C. oliveri as clearly distinct species. They have also agreed about the existence of C. harringtonia, but this widespread taxon is represented by a morphologically and genetically diverse suite of populations; there has been little agreement on its taxonomic circumscription. There has been considerable debate about the remaining taxa, C. alpina, C. koreana, C. griffithii, C. latifolia, C. lanceolata, C. mannii, C. nana, C. sinensis, and C. wilsoniana. The principal studies in this debate have been the morphological analyses by Cheng et al. (1978), Fu et al. (1999)), Farjon (2010), Lang et al. (2013), and Zhang et al. (2019), as well as molecular analyses by Hao et al. (2008), Ji et al. (2021) and Wang et al. (2022). The issue with the morphological studies is that "the morphological characters used for species delimitation are quite variable and characteristics usually overlap to a high degree between species resulting in a complex and controversial taxonomic history" (Wang et al. 2022). The earlier molecular analyses, however, were also inconclusive. The issue appears to have been resolved in the complete sequencing of the chloroplast genome by Wang et al. (2022), which yielded a phylogenetic tree represented by multiple specimens from all putative species and varieties (figure at right). That analysis found that chloroplast genes commonly used in phylogenetic studies were not adequate to resolve all taxa, but that sequencing of the complete chloroplast genome resolved the uncertainties with high confidence. The analysis identified 9 species, one of which is a previously undescribed cryptospecies. For the most part the new molecular classification agrees with that of Farjon (2010), except that it treats C. fortunei var. alpina at species rank, subsumes Farjon's C. latifolia within C. sinensis, and identifies the new cryptospecies (still undescribed as of mid-2024) within two samples that key out as Farjon's C. sinensis and are discussed on that page.
Finally, a note on nomenclature: in Latin, "Taxus" is a female noun, despite having the "-us" suffix typical of male nouns. Because of this, all botanical names based on Taxus (e.g. Austrotaxus, Cephalotaxus, etc.) are likewise female nouns, and the scientific names assigned to them must reflect that grammatical gender. E.g., the correct name is "Cephalotaxus harringtonia" rather than "Cephalotaxus harringtonii".
Description
Evergreen dioecious, rarely monoecious trees or shrubs. Branches opposite or whorled. Buds ovate, covered with numerous persistent imbricate scales. Leaves spirally arranged on terminal branchlets, appearing 2-ranked on lateral branchlets, needle-like, usually pectinate with a conspicuous midrib on the upper surface and 2 broad bluish stomata bands below, each with 11-24 rows of stomata; a single resin canal below the midrib. Pollen cones axillary, globose, on twigs formed the preceding year, subtended by 1 ovate bract, up to 1 cm diameter, each with 4-16 microsporophylls each bearing 2-4 sacs of nonsaccate pollen. Seed cones borne from axils of terminal bud scales, 1-8 per bud, long pedunculate, cones pendant, drupe-like, elliptic, 2-3 cm long, with a leathery, fleshy outer covering, green to reddish, containing 1-2 wingless seeds ripening the second season. Cotyledons 2. Chromosomes n = 12, among the largest of all conifer chromosomes (Silba 1986, Vidakovic 1991, Fu et al. 1999).
Fu et al. (1999) provide a key to the Chinese species, Farjon (2010) provides a key to the species and varieties he recognizes, and Lang et al. (2013) provide a key to the species that they recognize.
All Cephalotaxus appear to form vesicular-arbuscular mycorrhizas (Newman and Reddell 1987, cited by Brundrett 2008).
Distribution and Ecology
Korea, China, Japan, Burma, Laos, Viet Nam and India (Vidakovic 1991, Tripp 1995). The center of distribution is in China, which holds portions of the native range of a majority of species. Several of the species have largely disjunct ranges, while the ecological differences separating the principal widespread species (C. fortunei, C. mannii, and C. sinensis) remain unclear.
Distribution data for all species of Cephalotaxus, based on GBIF occurrence download https://doi.org/10.15468/dl.bz6u39 (2024.08.24). Open left pane for legend; click on any icon for a link to source information. See map notes (left pane) for details on map preparation. All taxa are shown here except C. lanceolata, which has only 5 located occurrences in Yunnan and adjacent Myanmar.
- C. alpina: China, at subalpine elevations in S Gansu, N and W Sichuan, and N and W N Yunnan. As shown on the map above, the Gansu occurrence is far from the other locales and appears doubtful.
- C. fortunei: Widespread throughout warm temperate and subtropical China east of about longitude 102°; reported from N Myanmar.
- C. hainanensis: Endemic to the mountainous interior of Hainan Island, China.
- C. harringtonia: Korea, Japan, Taiwan, and perhaps in neighboring portions of mainland China.
- C. lanceolata: Extremely local in China (NW Yunnan) and adjacent N Myanmar.
- C. mannii: China (Yunnan), India, Laos, Myanmar, Thailand, and Viet Nam.
- C. oliveri: China (Chongqing, N Guangdong, Guizhou, W Hubei, Hunan, E Jiangxi, S and W Sichuan, E Yunnan); widespread but uncommon.
- C. sinensis: Widespread in China (Anhui, Chongqing, Fujian, Gansu, Guangdong, Guangxi, Guizhou, Hainan, Henan, Hubei, Hunan, Jiangsu, Jiangxi, Shaanxi, Shanghai, Sichuan, Yunnan, Zhejiang), with broad distribution similar to C. fortunei.
All species are highly shade tolerant, typically growing as understory trees or shrubs in humid temperate to subtropical broadleaf forests, and are typically uncommon within their ranges. C. alpina and northern provenances of C. harringtonia can tolerate cold temperate climates (USDA Zone 5 or 6), but none can tolerate aridity, and most can be damaged by exposure to full sun. They seem to be highly browse-resistant due to unpalatability. There is some concern that they suffer from poor regeneration due to a slow reproductive cycle (about two growing seasons for both male and female full development), dioecious habit, slow growth, long distances between individual plants, and seed predation by birds and mammals (although such predation is probably the main agent of seed dispersal). All species are threatened in their native range, primarily due to habitat loss (Tripp 1995).
Remarkable Specimens
The largest trees are in C. harringtonia. I have no age data.
Ethnobotany
Due to the long-standing taxonomic controversies around the genus, many accounts of its use are not confidently assignable to particular species. In traditional use, most species were occasionally employed as a source of timber, but since it is generally a shrub or small tree and not found in continuous stands, this was uncommon. Like Taxus the wood is tough and durable, and was widely used for tool handles and household implements. A lamp oil was pressed from the seeds in India, Japan, and perhaps elsewhere. Cephalotaxus has a long history in traditional medicine; see Hao (2021) for a review focused on uses in mainland China. Uses seem to have included treatment of cough, internal bleeding, cancer (e.g. reduction of tumors), bruises, rheumatism, pain, ascariasis, hookworm, and scrofula; the plant was used by many ethnic groups such as the Miao, Yao, Dong, She, and Han. I have not found similar use documented in Korea, Japan and Taiwan, but it seems likely.
In modern times, nearly all uses of Cephalotaxus are medicinal, although some species are still subject to occasional timber harvest. All species have been found to contain anticancer alkaloids with names like cephalotaxine and harringtonine; the full list of such chemicals is extensive, many of them unique to one or two taxa. In some areas substantial numbers of plants have been stripped of their bark and foliage for the purposes of extracting the anticancer compounds. Such exploitation threatens the survival of several species, but there is reason to hope that this can be avoided (as it was with Taxus) by synthesizing the compounds (Tripp 1995, Farjon (2010)). The level of such use and the pharmacological importance of the respective compounds are discussed in the species accounts.
Observations
The species with the widest native ranges and greatest cold tolerance, C. fortunei, C. harringtonia, and C. sinensis, can be readily found in botanical gardens and arboreta throughout the cold-temperate West. However, other species are not represented in any U.S. or U.K. gardens and must be seen in their rapidly-vanishing native habitats.
Remarks
The name is derived from the Greek kephale, meaning "head", and taxus, referring to the head-like shape of the staminate flowers in yew (Vidakovic 1991).
Philipp Franz von Siebold sent the first Cephalotaxus to Europe from Japan, in about 1829 (Tripp 1995).
The fossil record includes Jurassic specimens in Greenland, and Europe and NW North America during the Miocene and Pliocene (Tripp 1995).
Citations
Brundrett, Mark. 2008. Mycorrhizal Associations: The Web Resource. mycorrhizas.info, accessed 2009.06.09.
Hao, Da-Cheng. 2021. Biodiversity, chemodiversity, and pharmacotherapy of Cephalotaxus medicinal plants. Pp. 243-304 in Da-Cheng Hao (ed.), Taxaceae and Cephalotaxaceae. Academic Press.
Ji, Yunheng, Changkun Liu, Jacob B. Landis, Min Deng, and Jiahui Chen. 2021. Plastome phylogenomics of Cephalotaxus (Cephalotaxaceae) and allied genera. Annals of Botany 127(5): 697-708.
Lang, Xue-Dong, Jian-Rong Su, Shu-Gang Lu, and Zhi-Jun Zhang. 2013. A taxonomic revision of the genus Cephalotaxus (Taxaceae). Phytotaxa 84(1): 1-24.
Majeed, Aasim, Amandeep Singh, Shruti Choudhary, and Pankaj Bhardwaj. 2019. RNAseq-based phylogenetic reconstruction of Taxaceae and Cephalotaxaceae. Cladistics 35(5):461–68.
Manchester, S.R., Z.D. Chen, A.M. Lu, and K. Uemura. 2009. Eastern Asian endemic seed plant genera and their paleogeographic history throughout the Northern Hemisphere. Journal of Systematics and Evolution 47:1–42.
Neger, F.W. 1907. Die Nadelhölzer (Koniferen) und übrigen Gymnospermen Leipzig (pp. 23, 30-31). Available at Google Books, accessed 2012.11.22.
Newman, E.I. and P. Reddell. 1987. The distribution of mycorrhizas among families of vascular plants. New Phytologist 106: 745-751.
Shi, Gongle, Zhi-Yan Zhou, and Zhiming Xie. 2010. A new Cephalotaxus and associated epiphyllous fungi from the Oligocene of Guangxi, South China. Review of Palaeobotany and Palynology 161:179–95.
Singh, H. 1961. The life history and systematic position of Cephalotaxus drupaceae Sieb. et Zucc. Phytomorphology 11:153-197.
Wang, Jie, Chao-Nan Fu, Zhi-Qiong Mo, Michael Möller, Jun-Bo Yang, Zhi-Rong Zhang, De-Zhu Li, and Lian-Ming Gao. 2022. Testing the Complete Plastome for Species Discrimination, Cryptic Species Discovery and Phylogenetic Resolution in Cephalotaxus (Cephalotaxaceae). Frontiers in Plant Science 13: 768810. https://doi.org/10.3389/fpls.2022.768810, accessed 2024.08.26.
Zhang, Jian-Wei, Ashalata D’Rozario, Xiao-Qing Liang, and Zhe-Kun Zhou. 2019. Middle Miocene Cephalotaxus (Taxaceae) from Yunnan, southwest China, and its implications to taxonomy and evolution of the genus. Palaeoworld 28(3): 381-402.
See also
Cheng Wan-chün, Fu Li-kuo & Chao Chi-son. 1978. Cephalotaxaceae. In: Cheng Wan-chün & Fu Li-kuo, eds., Flora Reipublicae Popularis Sinica 7: 423-436.
Yinger, Barry. 1989. Notes on Cephalotaxus, the plum yew. Bull. American Conifer Society 6(3):57-59.