Conservation Status

Abies fraseri
(Pursh) Poiret 1817
Common names
Fraser fir, southern balsam fir (Hunt 1993), balsam, she-balsam.
Taxonomic notes
It was long suspected and argued that Fraser fir was sister to Abies balsamea, but molecular data have established with high confidence that this taxon should be described as a subspecies or variety of balsam fir. Similarities between the taxa include:
- cone and needle morphology (Robson et al. 1993);
- resin chemistry (Thor and Barnett 1974);
- hybridization (Hawley and Dehayes 1985);
- nuclear microsatellite markers (Potter et al. (2010);
- chloroplast DNA (Semerikova and Semerikov 2014); and
- multilocus nuclear markers (Semerikova and Semerikov 2016).
The apparent variation between the two taxa is due to a latitudinal cline, with the northern populations of Fraser fir distinctive enough to have been described as a distinct taxon (Abies balsamea (L.) Mill. var. phanerolepis Fernald 1909), and the southern described as A. fraseri. Moreover, the treatment of A. fraseri as a good species has long been justified by a conspicuous difference in morphology: A. balsamea cones have included bracts, whilst those of A. fraseri are exserted and reflexed. Curiously, the same character has been used to justify a variety of A. magnifica on the west side of the continent. However, it now seems likely that the morphological changes observed along this cline are simply a response to environmental variation.
Nonetheless, there are some reasons to treat the two taxa as distinct at the species rank. A.E. Matzenko classified Fraser fir and balsam fir in different taxonomic series (Hunt 1993); similarly, Farjon (1990) assigns both species to Section Balsamea but places Abies balsamea in subsection Laterales and A. fraseri in subsection Medianae. These assignments, however, are based on morphological arguments that are not incompatible with the molecular evidence. I retain A. fraseri as a distinct species here mainly for convenience; at this writing (2020) few sources have yet accepted the unification of the two taxa. Since the differences between the taxa are consistent with a latitudinal cline, Fraser fir would best be treated as a subspecies, viz. Abies balsamea (L.) Mill. subsp. fraseri (Pursh) E. Murray 1982.
Synonymy (Farjon 1998):
- Pinus fraseri Pursh 1814;
- Pinus balsamea L. var. fraseri (Pursh) Nutt. 1818;
- Abies humilis Bach. 1826;
- Picea fraseri (Pursh) Loudon 1838
- Abies balsamea (L.) Mill. var. fraseri (Pursh) Spach 1841;
- Abies americana Prov. 1862 non Mill. 1768;
- Picea balsamea (L.) Loudon var fraseri (Pursh) J. Nelson 1866;
- Abies balsamea (L.) Mill. subsp. fraseri (Pursh) E. Murray 1982.
Description
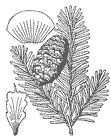
Trees to 25 m tall and 75 cm dbh, with a open, symmetrical, pyramidal to spire-shaped crown. Bark gray, thin, smooth, with age developing appressed reddish scales, later turning gray. Branches diverge from the trunk at right angles; twigs are opposite, pale yellow-brown, with a reddish pubescence. Buds exposed, light brown, conic, small, resinous, apex acute; basal scales short, broad, equilaterally triangular, glabrous, resinous, margins entire, apex sharp-pointed. Leaves 1.2-2.5 cm × 1.5-2 mm, 2-ranked, particularly in lower parts of tree, to spiraled, flexible; cross section flat, grooved on the upper side; odor turpentinelike, strong; lower surface with (8-)10(-12) stomatal rows on each side of the midrib; upper surface dark lustrous green, sometimes slightly glaucous, with 0-3 stomatal rows at midleaf, these more numerous toward leaf apex; apex slightly notched to rounded; resin canals large, ± median, away from margins and midway between upper and lower epidermal layers. Pollen cones at pollination red-yellow or yellow-green. Seed cones cylindric, 3.5-6 × 2.5-4 cm, dark purple overlaid with yellowish green bracts, sessile, apex round; scales ca. 0.7-1 × 1-1.3 cm, pubescent; bracts exserted and reflexed over cone scales. Seeds 4-5 × 2-3 mm, body brown; wing about as long as body, purple; cotyledons ca. 5. Wood pale brown with white sapwood. 2n=24. (Sargent 1922, Hunt 1993). See García Esteban et al. (2004) for a detailed characterization of the wood anatomy.
Distribution and Ecology
USA: Georgia, North Carolina, Tennessee, Virginia; the hybrid reported to also occur in West Virginia, in the Yew Mountains near Spruce Knob. Found at 1,200-2,038 m elevation in mountain forests, usually on north-facing slopes. Annual precipitation of 850-2,000 mm is distributed through the year, with heavy snowfall in the winter. There are many scattered populations, usually forming mixed stands with Picea rubens, sometimes Betula papyrifera, and an understory commonly depauperate, rich in mosses, or dominated by Ericaceous shrubs (Farjon 1990, Hunt 1993). See also Thompson et al. (1999). Hardy to Zone 4 (cold hardiness limit between -34.3°C and -28.9°C) (Bannister and Neuner 2001).
I have compiled the range map below using 81 herbarium records from the online database at the NCU Herbarium. These resulted in 48 verifiably different locations. I was not able to verify the identifications, but this is the only Abies native to North Carolina or Tennessee. Nearly every record states that the collection was made near the summit of the mountain, except for one record that identifies a collection in a bog or fen near Celo, NC.
Distribution data shown in red polygons are from USGS (1999).
Fraser fir has been decimated in many areas by the attacks of an introduced insect pest, the balsam wooly adelgid. The species is classified "vulnerable" due to the depredations of this pest. First introduced in New England in 1908, the adelgid reached Mt. Mitchell (highest point in the eastern United States and formerly home to a splendid Abies fraseri - Picea rubens forest) in 1957 and has since killed at least 80% of the mature Fraser firs on the mountain. The adelgid kills the tree by inducing a reaction in which the tree blocks sap flow in its xylem. The affected wood, called "rotholtz" (red heart) is very dense and has a red color (source: interpretive museum at Mt. Mitchell summit, 2004.10.25).
Remarkable Specimens
There are records of a tree 66.3 cm dbh and 12.22 m tall in September 2005, in Mount Mitchell State Park, NC; and also of a tree 36.4 cm dbh and 21.61 m tall in June 2007, in Great Smoky Mountains National Park, NC (Eastern Native Tree Society 2012).
The maximum age is known only from an unsupported figure of about 150 years (Burns and Honkala (1990).
Ethnobotany
Once upon at time: "The fragrant branches are popular with travelers, for beds" (Dallimore et al. (1967)). Due to its rarity and general decline, the species is no longer exploited. However, it has twice (as of 2024) served as the U.S. Capitol Christmas tree.
Several dendrochronology studies have examined growth decline and growth trends.
Observations
I have seen the tree on Clingman's Dome in Tennessee and near the summit of Mt. Mitchell. Mt. Mitchell is easily accessible by paved road; I think Clingman's Dome is too, but I was there in December and the trip involved quite a long walk on a road closed by drifting snow.
Remarks
The species is named for its discoverer, John Fraser (1750-1811), an ardent collector of North American plants, who first introduced it to European cultivation.
Citations
Britton, N.L., and A. Brown. 1913. Illustrated flora of the northern states and Canada. Vol. 1: 63. Image downloaded from the USDA-NRCS PLANTS Database, accessed 2004.12.23.
Eastern Native Tree Society. 2012. http://www.nativetreesociety.org/bigtree/maxlist/Max%20list%202.1.xls, accessed 2012.09.18, now defunct.
Farjon, Aljos. 1990. Pinaceae: drawings and descriptions of the genera Abies, Cedrus, Pseudolarix, Keteleeria, Nothotsuga, Tsuga, Cathaya, Pseudotsuga, Larix and Picea. Königstein: Koeltz Scientific Books.
Hawley, G. J. and D. H. Dehayes. 1985. Hybridization among several North American firs: 1. Crossability. Canadian Journal of Forest Research 15:42–49.
Murray, E. 1982. Notae Spermatophytae. Kalmia 12:23.
Poiret, J. L. M. 1817. Sapin - Abies, p. 35 in M. de Lamarck, Encyclopedie Methodique. Botanique, t. 5. Paris. Available at the Biodiversity Heritage Library, accessed 2020.01.17.
Potter, K. M., J. Frampton, S. A. Josserand, and C. Dana Nelson. 2010. Evolutionary history of two endemic Appalachian conifers revealed using microsatellite markers. Conservation Genetics 11(4):1499–1513.
Robson, K. A., J. Maze, R. K. Scagel, and S. Banerjee. 1993. Ontogeny, phylogeny and intraspecific variation in North American Abies Mill. (Pinaceae): an empirical approach to organization and evolution. Taxon 42(1):17-34.
Sakai, A. and C. J. Weiser. 1973. Freezing resistance of trees in North America with reference to tree regions. Ecology 54:118–126.
Semerikova, S. A., and V. L. Semerikov. 2014. Molecular phylogenetic analysis of the genus Abies (Pinaceae) based on the nucleotide sequence of chloroplast DNA. Russian Journal of Genetics 50(1):7–19.
Semerikova, S. A., and V. L. Semerikov. 2016. Phylogeny of firs (genus Abies, Pinaceae) based on multilocus nuclear markers (AFLP). Russian Journal of Genetics 52(11):1164–1175.
Thor, E. and P. E. Barnett. 1974. Taxonomy of Abies in the southern Appalachians: variation in balsam monoterpenes and wood properties. Forest Science 20:32–40.
See also
Elwes and Henry 1906-1913 at the Biodiversity Heritage Library. This series of volumes, privately printed, provides some of the most engaging descriptions of conifers ever published. Although they only treat species cultivated in the U.K. and Ireland, and the taxonomy is a bit dated, still these accounts are thorough, treating such topics as species description, range, varieties, exceptionally old or tall specimens, remarkable trees, and cultivation. Despite being over a century old, they are generally accurate, and are illustrated with some remarkable photographs and lithographs.
FEIS database.
Pursh, Frederick. 1814. Flora Americae Septentrionalis; or, A systematic arrangement and description of the plants of North America. London: White, Cochrane and Co. V. 2, P. 639. This is the first published description, available at the Biodiversity Heritage Library, accessed 2020.01.17.
The species account at Threatened Conifers of the World.