Podocarpaceae
Endlicher 1847
Common names
Podocarp family, 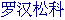 [Chinese].
[Chinese].
Taxonomic notes
In this treatment, 172 species in 20 genera:
The Podocarpaceae have been treated as an order: Podocarpales Pulle ex Reveal 1992, and several of its constituent genera, including Nageia, Phyllocladus and Saxegothaea, have been raised to the rank of Family by previous authors. Phyllocladus was traditionally thought to be the most atypical genus in the family, and it was often treated as Phyllocladaceae Bessey 1907; but molecular analyses (described below) have repeatedly shown Phyllocladus to be firmly rooted within one of the major clades of the Podocarpaceae.
The cladogram shown at right summarizes Podocarpaceae phylogeny as hypothesized by Biffin et al. (2011). Yellow lines show the principal clades; blue lines the inferred phylogeny on the basis of morphological, molecular and other (climate) characters; red lines the outgroup Araucariaceae; and green lines are error bars for each node (the 95% highest probability density divergence times). Note that Prumnopitys includes the two newer genera, Pectinopitys and Sundacarpus. The time scale shows estimated times of divergence using Bayesian-relaxed clock analysis of plastid DNA. The analysis supports the monophyly of Podocarpaceae genera. The timeline was consistent with the fossil record, especially in showing a divergence between Araucariaceae and Podocarpaceae about 205 million years ago, and in the times of appearance of major genera. However, recent fossil discoveries have moved the origin of the Podocarpaceae into the Permian, requiring a recalibration of molecular clock estimates with an Araucariaceae/Podocarpaceae divergence at about 280 million years ago (Khan et al. 2023). See section 4.4 of Khan et al. (2023) for a recent overview of the fossil record for Podocarpaceae and current hypotheses about the origin and migration of the various genera.
The analysis of Biffin et al. (2011) can be compared to that of Knopf et al. (2011), performed at about the same time and with a comparable level of detail. Knopf et al. produce several cladograms based on types of molecular data, as well as a consensus tree based on combined analysis of molecular and morphological data. These cladograms reveal uncertainties about the structure of the major clade that includes Prumnopitys, the placement of Saxegothaea, and the relationship between Microcachrys and Phaerospharea, but overall show remarkable consistency with the analysis of Biffin et al. with regard to relationships of genera within the family. Also, both Knopf et al. and Biffin et al. confirm in their analyses that the strongest morphological indicators of phylogenetic relationships within the Podocarpaceae are characters of leaf anatomy.
Many of the genera described here were originally lumped into the huge catchall genus Podocarpus or the also-very-large genus Dacrydium, and appear as such in much of the literature. Here is a brief and simplified chronology of how the podocarps have been subdivided at the generic rank:
| 1786 - Solander |
Dacrydium described. |
| 1788 - Gaertner |
Nageia described, begins a long and complex history (mostly 1840 to 1990) with the net result of species being moved from Podocarpus into Nageia. |
| 1807 - L'Heritier |
Podocarpus described, begins accumulating species, a process that continues today. |
| 1825 - Richard |
Phyllocladus described. No one ever confuses with it any of the other podocarps. |
| 1845 - Hooker f. |
Microcachrys described. |
| 1850 - Archer |
Pherosphaera described, lumping several new species with Microcachrys. |
| 1851 - Lindley |
Saxegothaea described. |
| 1861 - Phil. |
Prumnopitys described but is monotypic for 117 years. |
| 1903 - Pilger |
Acmopyle described. |
| 1969 - de Laubenfels |
Nageia species reassigned to the new genus Decussocarpus
Dacrycarpus
segregated from Podocarpus
Falcatifolium described, mostly with new species. |
| 1972 - de Laubenfels |
Parasitaxus described. |
| 1978 - de Laubenfels |
Seven Podocarpus species assigned to Prumnopitys. |
| 1982 - Quinn |
Halocarpus, Lagarostrobos, and Lepidothamnus segregated from Dacrydium. |
| 1987 - de Laubenfels |
Decussocarpus invalidated and its species reassigned to Nageia. |
| 1989 - Page |
Afrocarpus segregated from Nageia and Podocarpus
Retrophyllum segregated from Nageia
Sundacarpus segregated from Prumnopitys |
| 1995 - Molloy |
Manoao segregated from Lagarostrobos. |
| 2019 - Page |
Pectinopitys segregated from Prumnopitys. |
Description
Evergreen shrubs or trees, usually with straight trunk and more or less horizontal branches. Leaves usually spirally arranged, sometimes opposite, scale-like, needle-like, or more apart, flat and leaf-like, linear to lanceolate. Monoecious or dioecious. Pollen cones usually catkin-like; stamens numerous, close together, imbricate, each with 2 sporangia; pollen grains usually winged. Female cones maturing in one year, much reduced to a few fleshy bracts or scales, pendant, usually borne on a thin peduncle, containing a single inverted ovule. Seeds completely covered by a fleshy structure referred to as an epimatium, wingless. Epimatium and integument sometimes connate and forming a leathery testa. Cotyledons 2, with 2 parallel vascular bundles (Van Royen 1979, Silba 1986).
All investigated Podocarpaeceae have vesicular-arbuscular mycorrhizas (Newman and Reddell 1987, Brundrett 2008 and citations therein).
Distribution and Ecology
The family is predominantly found in the Australasian region, with most taxa native from New Zealand to SE Asia. Most of species are not of wide distribution, being confined to one or a few islands, viz. Tasmania, New Zealand, New Caledonia, New Guinea, Philippines, Borneo. Some species of the genera Dacrydium, Lepidothamnus, Nageia, Podocarpus, Prumnopitys and Saxegothaea are found beyond Australasia, in India, Japan, China, Africa, the Caribbean and the New World south from Mexico to Chile. Of these, Saxegothaea, a monotypic genus found in Chile and Argentina, is the only genus with no representatives in the Australasian region. Most members of the family are trees native to wet tropical or subtropical (often, tropical mountains) forests. A few are small trees or shrubs native to forest understory environments.
Worldwide distribution of Podocarpaceae, generalized to 1x1 degree cells; data from the BRAHMS database at Conifers of the World, accessed 2021.11.11.
Biffin et al. (2011) provide a thoughtful exploration of the relationship between conifers and angiosperms that is focused on Podocarpaceae, one of the few conifer groups that has continued to speciate and thrive within an angiosperm-dominated biome, specifically, the wet tropical forest. They find that, like most other conifer families, the Podocarpaceae originally had scale-like or needle-like foliage, but that adaptive radiation (genera Acmopyle through Podocarpus in the cladogram at right) during the past 65 million years has been characterized by the emergence of various types of flattened, often broad leaves, which now characterize most species in the family. This adaptation, which facilitates efficient light harvesting in shaded environments, has emerged during the time when the podocarps have been in direct competition with angiosperms, and likely explains why the podocarps have continued to prosper in wet tropical forests while most other conifers are excluded from the biome; the timing of this change also coincides with larger ecological and climatic changes that have been linked to evolution of tropical broadleaf habit in the angiosperms. It also appears that foliage flattening has evolved independently at least five times in the family. Although Biffin et al. don't mention it, the other gymnosperm genera found in the wet tropics, Agathis and Gnetum, have also developed flattened, broad leaves.
The Podocarpaceae are, in general, very much of conservation concern. The IUCN (2020) has identified 8 taxa as critically endangered, 27 as endangered, and 20 as vulnerable, thus 32% of all species in the family are at risk. Principal factors of decline, which typically affect all taxa regardless of their conservation listing status, include habitat conversion through agriculture or development, timber harvest, grazing, and climate change.
Remarkable Specimens
The largest volume (and by inference, biomass) is found in the totara, Podocarpus totara. This has been the largest for many years and is unlikely to be exceeded by any other species in the family. The largest known specimen is nearly 4 m in diameter, however, both larger and taller trees are known from the Cupressaceae, Pinaceae, and Araucariaceae.
The tallest is probably New Zealand's kahikatea, Dacrycarpus dacrydioides, at 62.7 m tall. However, there may be a sampling bias, as this species has been studied much more closely than many other podocarps. There is an unconfirmed report of an Afrocarpus falcatus 63 m tall, and Retrophyllum filicifolium is thought to achieve 60 m. For most taxa, there are no data. However, many species in the Podocarpaceae occur as emergents in tropical forest, which is reason to expect that they can achieve exceptional heights. The smallest species in the family is Lepidothamnus laxifolius; fruiting specimens barely 7.6 cm tall and wide have been found.
The Huon pine, Lagarostrobos franklinii, achieves an age of approximately 3,000 years, making it one of the oldest known of all conifers (see this table for comparison to other species). This great age is likely related to the unusual ecological setting of these trees, a very wet and cold alpine site in Tasmania; and may also be related to the fact that this is one ramet of an ancient male clone in an area with no female plants. Then there are a group of New Zealand species: Prumnopitys taxifolia has a reported age of 1358 years, and there is a rather vague report of a ring-counted age of 1200 years for Dacrydium cupressinum. There is an even more vague report of 1000 years (a nice round number) for Halocarpus biformis. Then we have a vague number of 750 years for Saxegothaea conspicua in South America, and a precise number of 744 years for two species, Afrocarpus falcatus in South Africa, and Pectinopitys ferruginea in New Zealand. Dacrycarpus dacrydioides in New Zealand reaches 716 years. Maximum ages of 400 to 700 years have been found in Manoao, Phyllocladus, and Podocarpus, and for the remaining genera, I have no data.
Ethnobotany
Like most conifers, the Podocarpaceae have recorded uses by native peoples and in the industrialized world, with the latter uses both economic and research-related. They have some pharmacological, dietary and spiritual importance, but have most often been exploited for their wood. However, all of these uses are less well-known in the Podocarpaceae than in the other large conifer families, for several reasons. First, the Podocarpaceae are often minor components of angiosperm-dominated forests, so they don't command the ecological or economic importance of conifers in conifer-dominated forests. Second, they are often minor or rare species in their own right, largely distributed on islands or on biogeographic islands such as mountaintops, where they are often subject to very limited human use; many species, for example, don't even have common names. Third, they primarily occur in less-developed countries and in less-studied habitats, so they attract less attention than the more celebrated taxa found in the Cupressaceae and Pinaceae. In settings where podocarps conform to the conifer norm of being large, ecologically dominant taxa in locations with appreciable human populations, they are generally important to human use and commerce. Their wood is exploited for many uses because it is generally of good quality and is often rot-resistant and attractive as well; however, most species are slow-growing, so there is an oft-repeated pattern of species being exploited for timber until only few and small trees remain, at which point they cease to be exploited. Often their habitat gets converted to agricultural use or timber plantations of exotic species, so that many podocarps are threatened or endangered species. Their bark and foliage extracts have been locally important in traditional medicine and in modern times are explored for pharmacological value. Their seed-bearing structures are sometimes exploited for food, or for usable oils. The species that occupy seasonal climates, and particularly temperate climates, are sometimes used in dendrochronological research, although most tropical species have proven unsuitable for various technical reasons.
Observations
See the generic and species descriptions.
Remarks
Netta E. Gray did much of the early work on Podocarpus and its segregates (Afrocarpus, Nageia, Prumnopitys, and Retrophyllum). She was accordingly honored by D.J. de Laubenfels with Podocarpus grayae de Laub. 1985. The September 1973 Plant Science Bulletin carried this brief biography of Mrs. Gray:
Netta E. Gray worked and studied with John T. Buchholz at the University of Illinois, completing her Masters degree in Botany. She was particularly interested in the genus Podocarpus. The work she started with Buchholz and later carried on herself was a careful systematic treatment of each of the sections of Podocarpus. She was concerned with the systematic importance of anatomical details, and the geographical distribution and evolution of the genus. As a result of her diligent study of the group she became one of the world's authorities on this genus and had such visitors as R. Florin come to her door. She lived with her husband Professor Stephen W. Gray near Atlanta, Georgia and taught at Agnes Scott College in Decatur, Georgia. She continued an active interest in research and publication about the genus, Podocarpus, as well as the Gymnosperms as a whole, until her death on August 24, 1970.
Besides Ms. Gray and Mr. Buchholz, the name of David J. de Laubenfels (1925-2016) is also prominent in the Podocarpaceae; he wrote extensively about the genus, and described many of the currently-recognized species and infraspecific taxa. For four decades his authoritative 1972 gymnosperm volume of the Flore de la Nouvelle-Caledonie et Dependances was the definitive treatment of the 43 endemic conifers on that amazing island. He worked almost exclusively in the tropics, ultimately describing over 100 taxa of tropical conifers (including a variety of Araucariaceae and a few Cupressaceae, as well as scores of podocarps), and his noteworthy publications extend from discovery of the only parasitic conifer (Parasitaxus) in 1959 through to a strong attempt to interpret the Podocarpus neriifolius species complex in 2015.
Citations
Brundrett, Mark. 2008. Mycorrhizal Associations: The Web Resource. mycorrhizas.info, accessed 2009.06.09.
IUCN. 2020. IUCN Red List version 2020-1: Table 4b: Red List Category summary for all plant classes and families. https://nc.iucnredlist.org/redlist/content/attachment_files/2020_1_RL_Stats_Table_4b.pdf, accessed 2022.09.23.
Khan, Raees, Robert S. Hill, Jie Liu, and Ed Biffin. 2023. Diversity, distribution, systematics and conservation status of Podocarpaceae. Plants 12(5):1171. https://doi.org/10.3390/plants12051171.
Newman, E. I. and P. Reddell. 1987. The distribution of mycorrhizas among families of vascular plants. New Phytologist 106: 745-751.
Page, Christopher N. 2019. New and Maintained Genera in the Taxonomic Alliance of Prumnopitys s.l. (Podocarpaceae), and Circumscription of a New Genus: Pectinopitys. New Zealand Journal of Botany 57(3):137–53. https://doi.org/10.1080/0028825X.2019.1625933.
See also
Barker, N. P., E. M. Muller, and R. R. Mill. 2004. A yellowwood by any other name: molecular systematics and the taxonomy of Podocarpus and the Podocarpaceae in southern Africa. South African Journal of Science 100:629-632.
Buchholz, J. T. and N. E. Gray. 1947. A Fijian Armopyle. Journal of the Arnold Arboretum 28:141-143. Available: Biodiversity Heritage Library, accessed 2021.12.19.
Buchholz and Gray 1948a, 1948b, and 1948c.
Gray, N. E. and J. T. Buchholz. 1948. A Taxonomic Revision of Podocarpus. III. The American Species of Podocarpus: Section Polypodiopsis. Journal of the Arnold Arboretum 29:117-122. Available: Biodiversity Heritage Library, accessed 2021.12.19.
Gray, N. E. and J. T. Buchholz. 1951. A Taxonomic Revision of Podocarpus. V. The South Pacific Species of Podocarpus: Section Stachycarpus. VI. The South Pacific Species of Podocarpus: Section Sundacarpus. Journal of the Arnold Arboretum Vol. 32:82-92. Available: Biodiversity Heritage Library, accessed 2021.12.19.
Gray, N. E. 1953. A Taxonomic Revision of Podocarpus. VII. The African Species of Podocarpus: Section Afrocarpus. Journal of the Arnold Arboretum 34:67-76. Available: Biodiversity Heritage Library, accessed 2021.12.19.
Gray, N. E. 1953. A Taxonomic Revision of Podocarpus. VIII. The African Species of Section Eupodocarpus, Subsections A and E. Journal of the Arnold Arboretum 34:163-175. Available: Biodiversity Heritage Library, accessed 2021.12.19.
Gray, N. E. 1955. A Taxonomic Revision of Podocarpus. IX. The South Pacific Species of Section Eupodocarpus, Subsection F. Journal of the Arnold Arboretum 36:199-206. Available: Biodiversity Heritage Library, accessed 2025.02.21.
Gray, N. E. 1956. A taxonomic revision of Podocarpus X. Journal of the Arnold Arboretum 37:160-172. Available: Biodiversity Heritage Library, accessed 2021.12.19.
Gray, N. E. 1958. A taxonomic revision of Podocarpus XI. Journal of the Arnold Arboretum 39:424-477. Available: Biodiversity Heritage Library, accessed 2021.12.19.
Gray, N. E. 1960. A Taxonomic Revision of Podocarpus. XII. Section Microcarpus. Journal of the Arnold Arboretum 41:36-39. Available: Biodiversity Heritage Library, accessed 2021.12.19.
Gray, N. E. 1962. A Taxonomic Revision of Podocarpus. XIII. Section Polypodiopsis in the South Pacific. Journal of the Arnold Arboretum 43:67-79. Available: Biodiversity Heritage Library, accessed 2021.12.19.
Gray, N. E. 1969. An Interpretation of Podocarpus in Time and Space. The Bulletin of Georgia Academy of Science 27: 144-147.
Hill, R. S. and T. J. Brodribb. 1999. Southern conifers in time and space. Australian Journal of Botany 47:639–696.
The authors provide an earlier version of the "flattened foliage in the wet tropics" hypothesis.
Kelch, D. G. 1998. Phylogeny of Podocarpaceae: comparison of evidence from morphology and 18S rDNA. American Journal of Botany 85(7):986–996. Available: https://doi.org/10.2307/2446365, accessed 2021.12.19.
Khan, Raees, Robert S. Hill, Jie Liu, and Ed Biffin. 2023. Diversity, distribution, systematics and conservation status of Podocarpaceae. Plants 12(5):1171. https://doi.org/10.3390/plants12051171.
Laubenfels, David J. de. 1959. Parasitic conifer found in New Caledonia. Science 130:97.
Laubenfels, David J. de. 1969. A revision of the Malesian and Pacific rainforest conifers, I. Podocarpaceae, in part. Journal of the Arnold Arboretum 50:315–369.
Laubenfels, David J. de. 1972. Flore de la Nouvelle Caledonie et Dependancees 4 Gymnospermes.
Laubenfels, David J. de. 1985. A taxonomic revision of the genus Podocarpus. Blumea 30:251–278.
Laubenfels, David J. de. 1988. Coniferales. P. 337-453 in Flora Malesiana, Series I, Vol. 10. Dordrecht: Kluwer Academic.
Laubenfels, David J. de. 2015. New Sections and Species of Podocarpus Based on the Taxonomic Status of P. neriifolius (Podocarpaceae) in Tropical Asia. Novon 24:133–152.
Turner, B. J. and L. A. Cernusak (eds.). 2011. Ecology of the Podocarpaceae in tropical forests. Washington, DC: Smithsonian Institution Scholarly Press. vii+207.











