"Range of the genus Agathis Salisb. Figures above the hyphen indicate the number of endemic species, that below the hyphen the total number of species" (de Laubenfels 1988).

Phylogenetic tree for the Araucariaceae, modified from Escapa and Catalano (2013) with added information on the New Caledonia Araucaria from Gadeul et al. (2012).

The 'Square Kauri', a large tree of Agathis australis in New Zealand. Observation platform at base of tree gives scale [C.J. Earle, 2003.03].

Foliage from the crown of the Square Kauri [C.J. Earle, 2003.03].

Branch abscision, common in Agathis, helps the trees to minimize their epiphyte load. This shows a tree 15 cm diameter [C.J. Earle, 2003.03].

Radial symmetry in new terminal growth on Agathis corbassonii in New Caledonia [Adam Black, 2014.06.01, Facebook post].

The type specimen of Agathis alba, Tab. 57 in Rumphius (1741).

Agathis lanceolata in habitat, New Caledonia [Adam Black, 2020.02.09, Facebook post].

Agathis montana in habitat near the summit of Mt. Panie, New Caledonia [Adam Black, 2014.12.15, Facebook post].

Agathis ovata in habitat, New Caledonia iNaturalist observation 38111819 [Joey Santore, 2020.01.30]

Foliage on the tree shown above iNaturalist observation 38111819 [Joey Santore, 2020.01.30]

Nearly mature female cone from an Agathis robusta tree in Yatton Park, New Zealand [C.J. Earle, 2003.03].

Agathis
Salisb. (1807)
Common names
Kauri [Maori] (Boland et al. 1985).
Taxonomic notes
Synonymy: Dammara (Rumph.) Lambert 1822; Salisburyodendron A. V. Bobrov et Melikyan 2006.
Taxonomic relationships within Agathis have been described in a series of publications extending over the past 50 years (Whitmore 1977, Bowen and Whitmore 1980, Page 1980, Whitmore 1980, Whitmore and Page 1980, de Laubenfels 1988, New South Wales National Parks and Wildlife Service 1998, Farjon 2010, Escapa and Catalano 2013). Most of these analyses were based largely on morphology of the pollen cone and leaf cuticle, as foliar morphology is highly variable and female cones, which disintegrate upon drying, are seldom preserved (Whitmore 1980). Work to date has shown a fairly high level of agreement in regard to the total number of taxa (19 species), a situation largely due to the high degree of endemism in the genus, though there has been a long history of lumping or splitting around the most variable and widespread species, A. alba. The distribution of most taxa is confined to a relatively limited space within the vast archipelago reaching from Sumatra and the Philippines to New Zealand, that comprises the genus' range. Earlier taxonomic investigations by several different authors (de Laubenfels 1988, Page 1980, Whitmore 1980, New South Wales National Parks and Wildlife Service 1998) attempted to provide an infrageneric classification, but the several schemes presented showed little agreement. Elucidation of this puzzle required molecular analysis, including rbcL gene sequences by Setoguchi et al. (1998) and integrated molecular, morphological and fossil data by Escapa and Catalano (2013), evaluating relationships within the Araucariaceae as a whole. The results of that analysis are shown in the phylogenetic tree at right, which establishes a close relationship to Wollemia and shows three primary branches corresponding to A. australis of New Zealand, the four species native to New Caledonia, and the remaining species scattered across Oceania. Although not all species were treated, it seems likely that A. corbassonii would fall into the New Caledonia clade, while A. flavescens, A. kinabaluensis, A. labillardieri, A. lenticula, A. orbicula, and A. silbae would fall into the extended Oceania clade. Further work is needed to resolve relationships within the latter group, which seems likely to have speciated over a fairly long period.
Taxa presented in this treatment include the following:
Description
Evergreen trees, usually monoecious, very large, with clear straight boles beneath a globular crown (young trees conical). Bark smooth and light grey to reddish, peeling with large thin irregular flakes that thicken on larger trees, leaving a pitted somewhat rough reddish brown surface, emits a milky sap when punctured. Branches horizontal or (when large) turning irregularly upward, leaving circular scars on the trunk after they fall. Buds usually globular, with few, imbricate, overlapping scales. Cotyledons 2, broad, lanceolate with an acute apex. Juvenile leaves larger than adult leaves, more or less acute, varying among the apecies from ovate to lanceolate. Adult leaves opposite, decussate, oval to linear, flat, broad, leathery, thick, with multiple parallel veins, sometimes glaucous beneath, with a short broad petiole, spirally arranged on main branchlets, opposite or alternate on side branchlets, reddish when young, turning dark green, leaving a rough cushion-like scar after they fall. Pollen cones appearing mostly on larger trees after seed cones have appeared, axillary (sometimes terminal), cylindrical, subtended at the base by rounded, sterile bud scales. Seed cones usually on short lateral branchlets, maturing and deciduous in 2 years, globose-ovoid, with numerous, flattened, broadly ovate scales without bracts and with minute apical umbos. Seeds more or less flattened and ovoid, one per scale, with 2 sometimes unevenly developed wings. Seed cone scales and seeds are asymmetrical with both dextral and sinistral forms produced (Silba 1986, de Laubenfels 1988). The wood is straight-grained, pale straw to yellow-brown, with a specific gravity of about 0.47 to 0.61 (Whitmore 1977).
Identification of the species of Agathis is most easily performed using a map: most species are not found in cultivation and have disjunct ranges. In confirmation of species identity, the most important characters are those associated with the pollen cone. Keys are provided by Whitmore (1980) and Farjon (2010). Farjon's key is more applicable to the species discussed here but cannot be reprinted here for copyright reasons. The following key is based on that of Whitmore (1980), modified using information from published descriptions; however it does not yet address the species A. lenticula and A. orbicula (mountains of N Borneo), or A. silbae (Vanuatu, Isla Espiritu Santo).
| | | |
| 1a | Head of microsporophyll with a distinct umbo, tessellately arranged in the cone | 2 |
| 1b | Head of microsporophyll not umbonate, imbricate in the cone. | 3 |
| 2a | Pollen cone at anthesis 6×11 - 8×16 mm; basal bracts clasping the cone; microsporophylls with stalk not winged, anthers inserted near head. Australia. | A. microstachya |
| 2b | Pollen cone at anthesis larger, 10×18-22 mm; basal bracts forming a loose shallow dish, full width of cone or projecting slightly; microsporophylls with broad papery wings extending 1-1.5 mm from head along stalk, anthers attached near the axial end of these wings. New Guinea. | A. labillardieri |
| 3a | Microsporophyll head lanceolate, margin crenate-serrate. New Caledonia. | A. moorei |
| 3b | Microsporophyll head roundish, margin entire or minutely erose. | 4 |
| 4a | Microsporophyll head narrowing gradually into stalk. Pollen cone a flexuous cylinder 9×60 mm at anthesis. Queensland, east New Guinea, New Britain. | A. robusta |
| 4b | Microsporophyll more or less abruptly joined to stalk. | 5 |
| 5a | Microsporophyll head normal to stalk, in adaxial view 1mm thick and 4 mm broad, in tangential view 4×4-5 mm, pale, chartaceous, margin serrulate. New Caledonia. | A. ovata |
| 5b | Microsporophyll head in adaxial view round or spatulate, only 1-3 mm broad (4-5 mm in A. borneensis). | 6 |
| 6a | Microsporophyll head large, 4-5 mm across at anthesis. Pollen cone with distinctly convex sides, 14-18 mm diameter at anthesis and ultimately attaining 20 mm. Sumatra, Malaya, Borneo. | A. borneensis |
| 6b | Microsporophyll head small, 1-3 mm across at anthesis. | 7 |
| 7a | Leaves small, ovate-lanceolate to lanceolate, 15×10 - 60×15 mm. Pollen cone with 2 of the basal bracts leaf-like. Microsporophyll head thick and small, 2×1.5 mm. Lowland forests. New Zealand. | A. australis |
| 7b | Leaves larger except in some mountain trees, relatively broader. Pollen cones without leaf-like bracts (except A. flavescens). Microsporophyll head laminate, at least towards margin. | 8 |
| 8a | Microsporophyll head tiny, 0.6 mm diameter at anthesis, entire. Pollen cone 4×7-9×16 mm. Australia. | A. atropurpurea |
| 8b | Microsporophyll head 1.5-2 mm diameter at anthesis. Pollen cone 6×12-10×60 mm. | 9 |
| 9a | Pollen cone 6×25-10×50 mm. | 10 |
| 9b | Pollen cone 6×13-10×25 mm. | 13 |
| 10a | Pollen cone with 2 of the basal bracts enlarged and leaf-like. | 11 |
| 11a | Pollen cone basal bracts to 10x30 mm, seed cone scales with a boss on the upper margin. Peninsular Malaysia. | A. kinabaluensis. |
| 11b | Pollen cone basal bracts to 4x20 mm; seed cone scales lacking a boss on the upper margin. Mountains of Borneo. | A. kinabaluensis. |
| 10b | Pollen cone without leaf-like bracteoles; peduncle short. New Caledonia. | 12 |
| 12a | Microsporophyll head at anthesis 2 mm across, lightly dentate. Pollen cone 8×40-10×60 mm. Mt. Panie, New Caledonia. | A. montana |
| 12b | Microsporophyll head at anthesis 1.5 mm across, serrulate to crenulate. Pollen cone 6×25-10×50 mm. New Caledonia. | A. corbassonii |
| 13a | Basal bracts of male cone forming a firm cupule narrower than cone base. New Caledonia. | A. lanceolata |
| 13b | Basal bracts forming a loose cupule, at least as wide as cone base except sometimes in A. alba. | 14 |
| 14a | Microsporophyll head at anthesis 2(-2.5) mm wide ×2 mm long, with a thin margin; anthers 6-14. Pollen cone 8×20-10×25mm at anthesis; cupule wider than cone base. Melanesia. | A. macrophylla |
| 14b | Microsporophyll head roundish, to 2 mm across, at anthesis, without a distinct thin margin; anthers 3-6. Pollen cone 6×12-8×20 mm at anthesis, ultimately 10 mm wide; cupule variable. Philippines, Celebes, Moluccas, some Bornean mountains, widely cult. in Java. | A. alba |
| | | |
Distribution and Ecology
Peninsular Malaysia to New Zealand, including Malesia, the Philippines, New Guinea, Melanesia and Australia. Their range extends from 10°30' N to 38° S, and from 96° E to 180° E. They occur from near sea level to about 2500 m elevation, and in lowland situations they are found on diverse substrates including podzolized sands, ultramafics, carbonates and silicates. They often occur as discrete emergent trees, but sometimes form pure stands or are mixed in the lower canopy (Whitmore 1977).
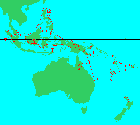
Worldwide distribution of Araucariaceae, generalized to 1x1 degree cells; cells are color-coded by genus, with yellow showing Agathis and green showing co-occurrence of Agathis and Araucaria; data from the BRAHMS database at Conifers of the World, accessed 2021.11.11.
The IUCN (2021), in assessments made from 2010 to 2014, assessed the status of 16 of the 19 species of Agathis and ranked 1 as "Least Concern", 4 as "Near Threatened", 5 as "Vulnerable", 5 as "Endangered", and 1 as "Critically Endangered". In general, deforestation and logging are the principal threats, although several of the species have always been vulnerable to disturbance due to their narrowly endemic distributions (primarily in New Caledonia and on Mt. Kinabalu, Borneo).
Agathis is predominately a genus of the tropical rainforests; most species thrive in sites that never see frost, and that receive between five and ten meters of rain per year. In these sites it grows often as solitary trees but more commonly in groves mixed with other rainforest trees. When young the trees have a robust growth and a pyramidal crown in the classic Araucarian growth model, but soon they become emergents, often extending ten or twenty meters into the sunshine above the tropical rainforest canopy, and here the monopodial trunk ends and the tree develops a swelling and complex crown reminiscent more of an oak than of a proper conifer. Few people would call Agathis a conifer on first acquaintance, because it grows in a forest that typically contains no conifers, because of its flat and often broad, leathery leaves, and because its few cones disintegrate on the tree and are rarely seen upon the ground.
Agathis primarily grows as a minor component of relatively inaccessible tropical forests, and most of the species resemble each other very closely. For these reasons it was one of the last groups of conifers to receive a reasonably complete inventory, with only two species described by 1830, another 10 between 1883 and 1916, and the last eight between 1940 and 1988. Fully 80% of the genus has only been described in the years since 1900, so that Agathis could be described as the last major group of conifers to be discovered by modern botany. It would be more fair, though, to say that two species, A. australis and A. alba, stood for the whole genus during most of the time between discovery and 1900.
Among these two, A. australis, native to northern New Zealand and the most temperate species of the genus in its climate preferences, was discovered even before the island was settled and quickly became the focus of a substantial logging industry; by the time the species was described in 1829, it had been exploited for naval stores (masts and spars) for nearly 50 years (Adams 1986). Exploitation of Agathis resins also began early in New Zealand.
A. alba, on the other hand, stands for the first Agathis to attract European attention. An Agathis, probably either A. alba or A. borneensis, was early noticed and exploited by representatives of the Dutch East India Company, and was described to science in 1741 by Rumphius as Dammara alba. This was before Linnaeus’ 1753 publication of the Genera Plantarum, and the genus name Dammara was subsequently found invalid, the earliest valid name, Agathis, being published in 1807. Nonetheless, the great tree named by Rumphius turned out to be the predominate tropical species of Agathis, and was soon being exploited for its timber and its resins throughout most of its range. Though many other names were applied to A. alba in subsequent years, no one really made any progress in systematically differentiating the species of Agathis until Warburg in 1900, and later, de Laubenfels in the 1970s and 1980s. These two botanists described most of the species now recognized, established that the principal taxonomic characters in the genus concern those of the pollen cone, and showed that most of the species have geographically separate distributions.
Remarkable Specimens
In contrast to most other good-sized conifer genera, most species of Agathis form large trees; many species are the largest trees found in their respective forest ecosystems. The largest, and one of the largest of all conifers, is Agathis australis. It also likely attains the greatest ages.
Ethnobotany
Thoughout its range, Agathis is highly sought after as a source of attractive, straight-grained, easily worked timber (Whitmore 1977). Due to its relative scarcity and premium value, it has now been largely logged out and current production is almost wholly derived from plantations.
The inner bark exudes a translucent or white resin, soluble in alcohol, called Manila Copal. This resin was formerly required for the production of many varnishes and of linoleum, and was harvested at the rate of 15-20,000 tonne per year from the 1920s to 1940s; however, the market has now been almost wholly replaced by synthetic substitutes (Whitmore 1977). Its memory lives on in the Kauri-Butanol rating system, which is a standardized measure of the solvent power of organic solvents based on their interaction with a solution of copal in butanol.
Agathis australis has been used in dendrochronology, but the other species have largely been problematic.
Observations
See the species accounts.
Remarks
Agathis is Greek for a ball of thread, an allusion to the globose female cone. Kauri is a Maori word, applied by that people to Agathis australis and generalized in modern usage to all species of Agathis (Boland et al. 1985).
Based on data from several wide-ranging species, Agathis participates in vesicular-arbuscular mycorrhizae; one associate is the phycomycete Endogone (Whitmore 1977).
Known pests include the seed-eating moth Agathiphaga, found in Queensland and the New Hebrides. In Queensland it is attacked by the kauri coccid, coniferococcus agathidis, a defoliator which can weaken the tree, leaving it open to attack by boring beetles such as Euthyrrhinus meditabundus. The kauri thrip Oxythrips agathidis is another, somewhat less destructive defoliator also known from Queensland. Naturally, various species are also attacked by a wide range of fungal diseases (Whitmore 1977).
Citations
Adams, J. G. Erne. 1986. Kauri: a king among kings. 2nd rev. ed. Auckland: Wilson & Horton Ltd. ISBN 0-86864-080-8.
Bobrov, A. V. and Melikyan. 2006. Komarovia 4:62.
Escapa, I. H., and S. A. Catalano. 2013. Phylogenetic analysis of Araucariaceae: Integrating molecules, morphology, and fossils. International Journal of Plant Sciences 174(8):1153–1170.
IUCN. 2021. Download of data summarizing conservation status for species of Agathis, https://www.iucnredlist.org/search?query=agathis&searchType=species, accessed 2021.11.25 18:23:26.
New South Wales National Parks and Wildlife Service. 1998. Wollemi Pine (Wollemia nobilis) recovery plan. Hurstville, NSW: New South Wales National Parks and Wildlife Service. 69p.
Page, C. N. 1980. Leaf micromorphology in Agathis and its taxonomic implications. Plant Systematics and Evolution 135: 71-79.
Rumphius, G. E. 1741. Herbarium Amboinense, V. 2. Amsterdam: Joannes Burmannus. Available: www.botanicus.org, accessed 2009.11.14.
Salisbury, Richard Anthony. 1807. Characters of several Genera in the Natural Order of Coniferae. Transactions of the Linnean Society. 8:308-317. Available: www.botanicus.org, accessed 2009.11.14.
Whitmore, T. C. and C. N. Page. 1980. Evolutionary implications of the distribution and ecology of the tropical genus Agathis. New Phytologist 84: 407-416.
See also
Mabberly, D. J. 2004. The Coming of the Kauris. Curtis's Botanical Magazine 19(4): 252-264. Abstract: Discusses the introduction to Europe of kauris, grown there as greenhouse ornamentals. Explains the importance of Sir Joseph Banks, Kew and the RBG Sydney, and the historical significance of kauris still growing in Sydney.
Stockey, R. A. and I. Atkinson. 1993. Cuticle micromorphology of Agathis Salisbury. International Journal of Plant Science 154: 187-225.











